Byline: Element: Iron (Fe). Atomic number: 26
The basis of all magnetic science started with iron. It is present in most permanent and soft magnetic materials that Quadrant designs and manufactures. It is abundant in nature, the 4th most common element by number and 1st most common by mass; making it relatively inexpensive. Humans have thousands of years of experience working with iron. Due to its strength, abundance, and low melting point it was easy to extract, forge, and maintain. Without some layer of protection (a coating for example), iron will bond with oxygen and hydrogen (air, water and other molecules) and create rust [FeO(OH), Fe(OH)3].
Iron is ferromagnetic; the strongest type of magnetism, named for iron (ferrum in Latin) because of iron’s magnetic strength. This was discovered over 2,000 years ago by studies of a stone found in nature called the lodestone (which was Fe3O4, a stable version of iron oxide called magnetite found in certain rocks) leading to the study of magnetism in physics we have today. Iron, causing the ferromagnetism in a lodestone, is magnetic because of the atom itself possessing a “magnetic moment”; the property of an atom or molecule that interacts with an applied magnetic field to give a mechanical moment (it moves when a magnetic field is present).
This permanent magnetic moment is not present in most elements and comes from the spin and placement of their electrons (every atom contains at least a neutron, proton, and electron). The electrons in an atom orbit the neutrons and protons, spinning while they do so. This moving electron (containing a known negative charge) creates an electromagnetic field while moving around the nucleus (all moving charges create an electromagnetic field, this is not exclusively for atoms with magnetic moments). If this field is not properly balanced with other electrons and moving charges, a net magnetic field can be measured. Iron has a permanent magnetic moment meaning the atom itself is a tiny electro-magnet because of the moving electron configuration (orbit and spin). There are only a few elements with this permanent magnetic moment property at room temperature.
When stacked in a molecule (such as multiple iron atoms, steel, or a magnet), the interatomic force of different atoms must keep the magnetic moments of many atoms parallel to each other. If the other atoms with iron have permanent magnetic moments facing another direction or interfere with iron’s magnetic moment, the end molecule might not be ferromagnetic (for example, 300 series stainless steel is mostly non-magnetic, or paramagnetic). See Figure 1 below for an example of the interatomic force keeping particles with magnetic moments unaligned (left) cancelling each other’s magnetic moment and aligned (right) adding their moment’s together. Bringing, even a large chunk of iron, to a certain heightened temperature will cause the interatomic bond to act differently (the magnetic moments of the iron atoms will suddenly face different directions and cancel each other out). This temperature is known as the Curie temperature (1043 Kelvin for Fe).
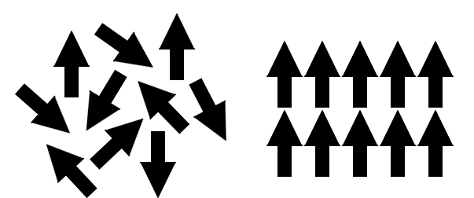
Figure 1 - This is a diagram showing the magnetic moments position while misaligned and interatomically parallel. CC BY-SA 3.0 by ACGrain 2012-01-29 Iron is an essential element in the study of magnetism and is present in most magnetic materials, both permanent and soft. It is inexpensive, easy to manipulate, and with the right corresponding atoms can help create the strong permanent magnet materials we have today.
Become a magnet pro!
Contact us to learn more about the magnetic materials that Quadrant has to offer.
